Introduction – From IV Drips to In-Body “Drug Depots”
Systemic chemotherapy works—but at a brutal cost. Life-saving drugs surge through the bloodstream, yet that same flood triggers fatigue, nausea, hair loss, immune crashes, and soaring medical bills. The shotgun approach of intravenous (IV) chemo is the price we pay for imprecision.
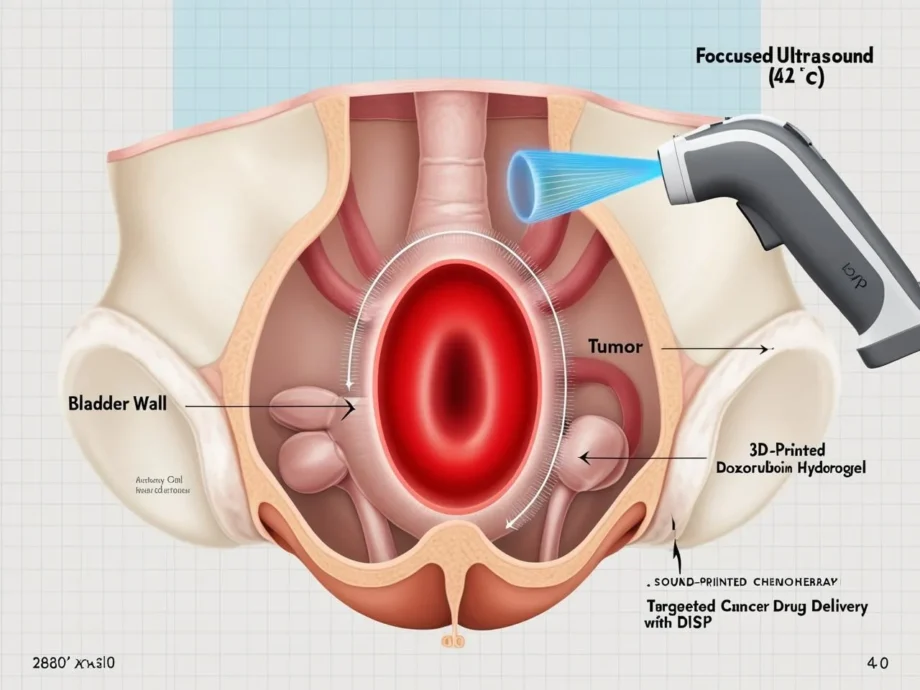
Caltech’s newly unveiled Deep-Tissue In Vivo Sound Printing (DISP) platform could change the equation. By “printing” doxorubicin-packed hydrogels inside the body at tumor-exact coordinates, DISP turns chemotherapy from carpet bombing into a precision strike. In mouse-bladder studies, 3D-printed drug depots killed tumor cells dramatically while sparing healthy tissues .
In this guide you’ll learn:
- How DISP works—from focused ultrasound “pens” to temperature-sensitive liposomes
- What the bladder-tumor experiment revealed about efficacy and safety
- Five key advantages over conventional chemo—and the hurdles still ahead
- A realistic roadmap to human trials and why personalized oncology could soon include ultrasonic 3D printing
Throughout, we naturally weave phrases such as targeted cancer drug delivery, 3D printed drug gels, and doxorubicin DISP so clinicians, researchers, and tech enthusiasts can easily find (and share) this breakthrough.
Why Precision Matters in Targeted Cancer Drug Delivery
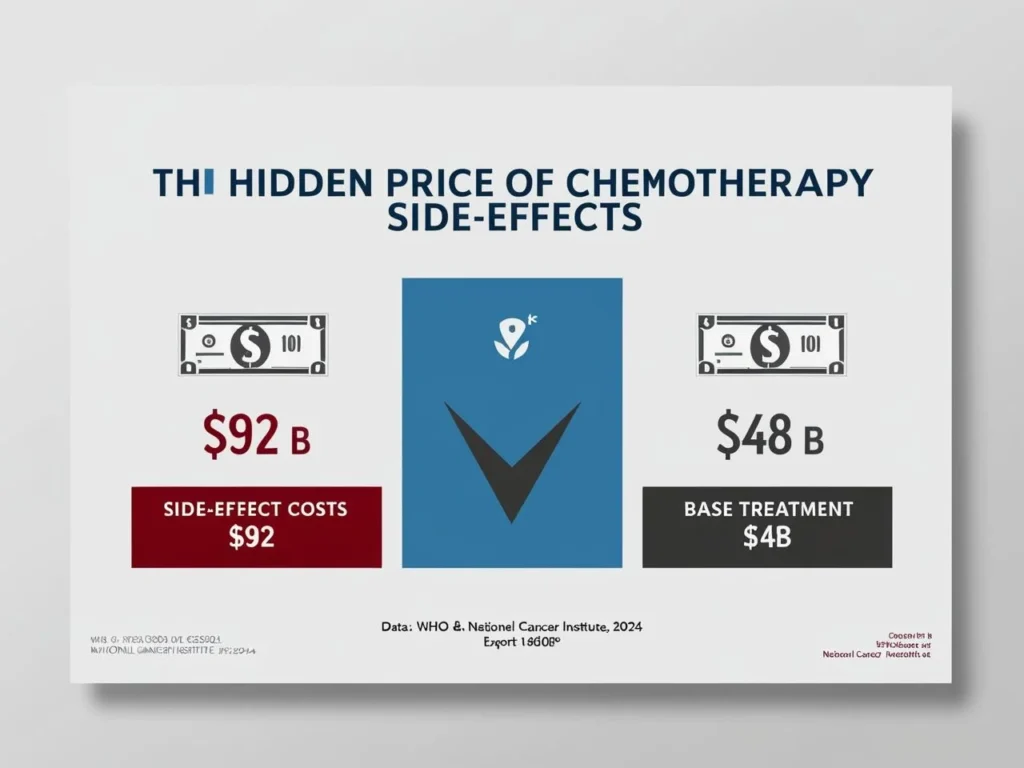
1 – Systemic Toxicity Is Costly—Medically and Financially
- Chemotherapy side-effects add billions in global costs and lost productivity each year. The National Cancer Institute now labels this burden “financial toxicity.”
- WHO and NCI analyses attribute steep price tags to supportive medications, extra hospitalizations, and lost income.
2 – Today’s “Local” Methods Still Miss the Mark
- Drug-eluting beads work for liver tumors but are limited by vascular access.
- Intravesical instillations for bladder cancer wash out within hours, pushing recurrence rates to ≈ 70 % within three years when surgery is the sole local therapy.
- Thermal intravesical chemo helps but still lacks sub-millimeter precision.
3 – The Promise of Printing in Place
A method that deposits chemo only where malignant cells live could slash side-effects, lower doses, and keep drugs in contact with tumors for days—not minutes.
DISP 101 – Ultrasound-Guided 3D Printed Drug Gels
| Core Component | What It Does | Why It Matters |
|---|---|---|
| Focused Ultrasound (FUS) | Invisible “pen” converging acoustic energy a few millimeters beneath tissue, raising temperature ~5 °C | Penetrates several centimeters—far deeper than near-infrared light |
| Low-Temperature–Sensitive Liposomes (LTSLs) | Nanoscopic fat bubbles pre-loaded with cross-linker and doxorubicin | Stable at 37 °C; burst at ≈ 42 °C, releasing cargo exactly at the FUS focal point |
| Gas-Vesicle Contrast Agents | Hollow bacterial-protein shells, highly echogenic | Make the bio-ink visible and change contrast once gelation occurs—providing real-time confirmation |
| Polymer Precursor (e.g., Alginate) | Printable “ink” that solidifies into a hydrogel | Conforms to tissue contours, trapping the drug depot in place |
Visualize a surgeon steering a stylus. Instead of ink on paper, it’s ultrasound through skin, drawing 3D shapes only the tumor can feel.
Sources: Davoodi E. et al., Science (2025); Reuters Health Rounds, May 9 2025 .

Case Study – Doxorubicin DISP in Mouse Bladder Tumors
| Step | Detail |
|---|---|
| Model | Orthotopic bladder tumors in immunocompetent mice |
| Bio-Ink Injection | 10 µL alginate solution with LTSLs (cross-linker + 0.5 mg/mL doxorubicin) + gas vesicles |
| Sound Printing | FUS raised local temp by ≈ 5 °C for ~10 s, drawing a 2 mm³ hydrogel ring around the tumor |
Results (Day 7)
- Tumor-cell apoptosis: 65 % TUNEL-positive vs 30 % with direct drug injection
- Drug retention: Doxorubicin signal persisted > 72 h in printed gels vs < 8 h in solution
- Systemic safety: No weight loss; liver/kidney histology normal
“We saw substantially more tumor cell death for several days with no observable systemic toxicity.” — Wei Gao, Caltech lead investigator
Source: UCLA CNSI press release, May 12 2025 .
Five Advantages of DISP Over Conventional Chemo
- Pin-Point Localization → Lower Systemic Dose
Doxorubicin AUC in plasma dropped ≈ 80 % in printed-gel mice. - Greater Depth & Versatility
FUS reaches centimeters; light-activated gels fade after 1–2 mm . - On-Demand 3D Shapes
Operators “draw” rings, patches, or cylinders that hug irregular cavities—ideal for bladder walls or post-resection margins. - Real-Time Ultrasound Feedback
Gas-vesicle contrast flips guesswork into see-and-tweak, slashing mis-targeting risk. - Upgradeable Cargo
Swap doxorubicin for cisplatin, add checkpoint inhibitors, or embed living immune cells—the chemistry is modular.
(Sprinkle synonyms like ultrasound drug printing, localized chemo gel, and acoustic bioprinting to capture varied search intent.)
Current Limitations & Safety Hurdles
| Challenge | Why It Matters | Path Forward |
|---|---|---|
| Off-Target Polymerization | Patient movement could trap gel in healthy tissue | Motion-tracking FUS + anesthetic protocols |
| Combination-Product Regulation | FDA will review device + drug + biomaterial as one | Early CBER/ODE engagement; predicate FUS devices ease the path |
| Unknown Long-Term Fate of Gas Vesicles | Potential delayed immune response | Chronic large-animal studies; engineer hypo-immunogenic variants |
| Thermal Overshoot | > 43 °C can cause vascular damage | Closed-loop thermometry + AI power modulation |
| GMP Bio-Ink Scale-Up | Liposome batches must meet strict specs | Partner with liposome CMOs and ink specialists |
Mouse histology showed minimal inflammation at 28 days , but rodent data ≠ human data—long-term studies remain essential.
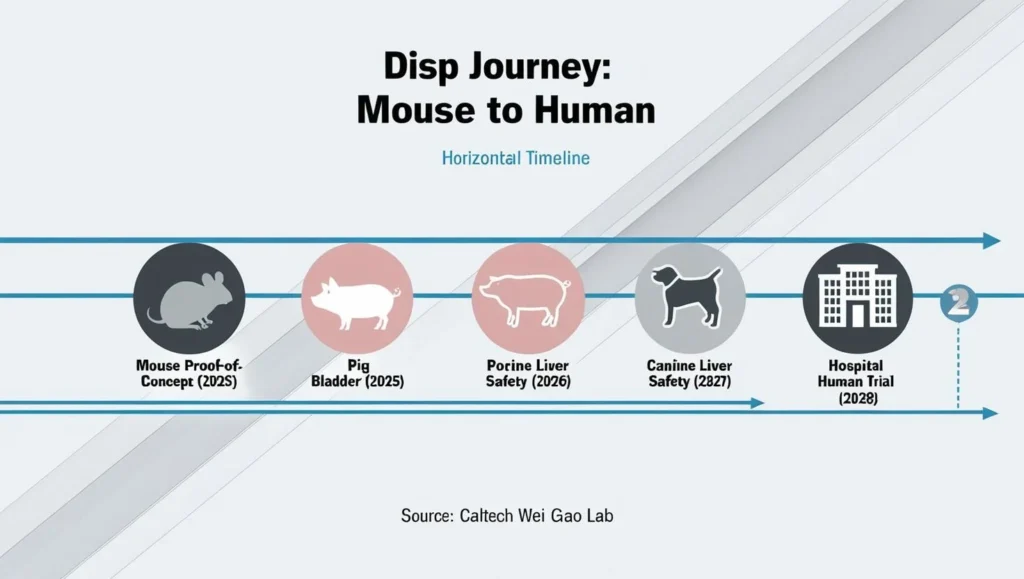
Roadmap to the First Human Trial
- Large-Animal Validation
- Porcine bladders (size ≈ human) in perfused models
- Canine liver tumors to test moving-target accuracy
- Hardware Scaling
- Combined FUS + MRI coils for thermometry
- Robotic arms to offset respiratory motion
- Manufacturing Milestones
- GMP-grade alginate + LTSLs in pre-filled syringes
- Single-use acoustic coupling pads for sterile OR workflow
- Regulatory Steps
- Pre-IND meeting → toxicology package
- IND filing → Phase I safety (≤ 12 patients)
- Endpoints: local toxicity, PK, preliminary tumor response via MRI
- Potential Breakthrough Device designation
Timeline: “If large-animal studies confirm safety, we aim for a first-in-human bladder-cancer trial within three years.” — Wei Gao
Future Outlook – DISP & the Next Era of Personalized Oncology
- AI-Steered Ultrasound: Deep-learning models predict organ motion (heartbeat, breathing) and auto-adjust FUS within milliseconds.
- Combo Therapies: Hydrogels co-loaded with doxorubicin and nivolumab could transform “cold” tumors into immune-hot targets.
- Robotic Endoscopy Integration: Flexible scopes inject bio-ink into pancreatic or colorectal lesions, then fire FUS from a tip-mounted probe.
- Market Potential: Analysts expect localized drug-delivery devices to grow at ≈ 12 % CAGR through 2030, driven by oncology and regenerative medicine demand.
FAQs
DISP is a new technique that uses focused ultrasound to “print” chemotherapy-loaded hydrogels directly onto tumors inside the body. It enables precise, localized drug delivery while minimizing side effects common with systemic chemotherapy.
Unlike traditional chemotherapy, which floods the whole body, DISP deposits drug-filled hydrogels exactly at the tumor site. This lowers systemic toxicity, improves drug retention, and increases tumor-cell death while sparing healthy tissues.
Early animal studies show promising results with high tumor kill rates and minimal systemic side effects. However, human trials are still pending, and more testing is needed to confirm long-term safety and regulatory approval.
DISP may be used for bladder, liver, and potentially pancreatic or colorectal cancers. Its precision and ability to adapt to complex tumor shapes make it especially promising for hard-to-treat or surgically challenging cancers.
Focused ultrasound acts like a pen that heats specific spots inside the body by just a few degrees. This heat causes special drug carriers (liposomes) to release chemotherapy at exact locations, forming a drug-loaded gel that sticks to tumors.
Benefits include precise localization, longer drug retention, reduced need for high doses, real-time feedback via ultrasound, and the ability to customize the shape and drug combination for each tumor.
If large-animal trials prove safe and effective, human trials could begin within the next 2–3 years, starting with bladder cancer patients. Regulatory milestones and manufacturing scale-up are in progress.
Conclusion – From Lab Mice to Cancer Clinics
Systemic chemo will remain essential, but its collateral damage is undeniable. 3D printed drug gels via DISP offer a tantalizing future: delivering the right dose to the right spot for the right duration—and nowhere else.
Mouse studies show dramatic tumor kill with minimal toxicity. The journey ahead winds through larger animals, regulatory checkpoints, and manufacturing scale-up, yet the destination is clear: a world where targeted cancer drug delivery is literally printed inside the patient, guided by the quiet hum of ultrasound.
References
- Davoodi E. et al. “Imaging-guided deep tissue in vivo sound printing.” Science (2025).
- Reuters Health Rounds. “Ultrasound triggers experimental 3D drug-delivery implants.” 9 May 2025.
- UCLA CNSI Press Release. “Caltech team prints chemotherapy inside living tissue.” 12 May 2025.
- National Cancer Institute. “Financial Toxicity and Cancer Treatment.” Updated 2024.
- Herr H. W. et al. “Impact of intravesical chemotherapy on recurrence rate of superficial bladder cancer.” J Urol (2001).
- Gofrit O. N. et al. “Chemo-thermotherapy for high-grade NMIBC.” Int J Hyperthermia (2019).