Imagine a future where implanted medical devices—stents, neural stimulators, or drug‑release systems—never need a battery replaced. Where the implant itself harvests energy from your own body’s fluids and receives wireless power through tissue. That is the bold vision behind a newly reported breakthrough from researchers at RMIT University, who have 3D‑printed a diamond–titanium hybrid implant that can both convert flowing liquid into electricity and receive external wireless power.
This article takes you inside the science, the challenges, and the transformative potential of this “battery‑free implant” concept. We’ll explore how it works, why diamond and titanium make a powerful combination, how the device is fabricated, what applications lie ahead, and what hurdles remain before this becomes clinical reality.
The RMIT Breakthrough: A New Kind of Implant That Harvests Energy
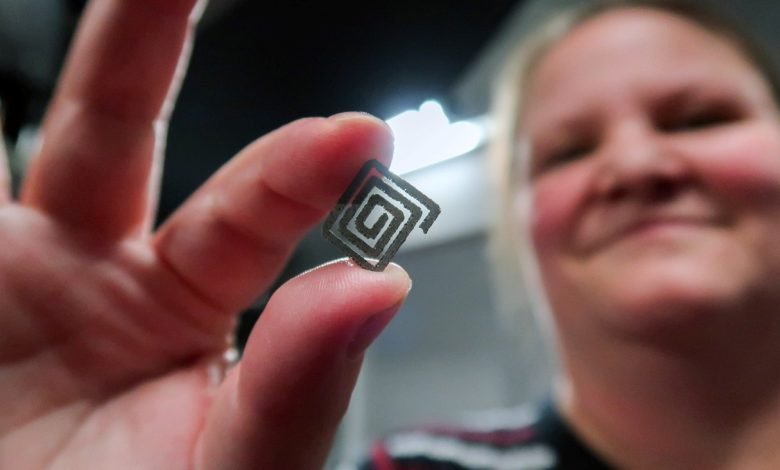
In 2025, RMIT researchers developed a 3D‑printed diamond–titanium device that can generate electricity from flowing liquid and receive wireless power through tissue—without embedding active electronics in the implant portion itself.
Dr. Arman Ahnood of RMIT’s School of Engineering explained their motivation: one of the biggest limitations in implant technology is the battery—batteries take up space, eventually degrade, and often require surgical replacement. In lab experiments, flowing saline over the hybrid surface produced a small but steady electrical signal. Embedding semiconductive diamond particles into a titanium matrix transforms what would be a passive structural component into something structural, biocompatible, and electrically active.
That combination—mechanical strength, biocompatibility, and dual energy harvesting + wireless reception—marks a significant step forward for next-generation medical implants.
How It Works: From Flowing Liquid to Electrical Power
To understand how this implant can generate electricity from liquid flow, we must look at two related physical phenomena: the triboelectric effect and the tribovoltaic effect.
The Triboelectric Effect
The triboelectric effect is what you see when you rub a balloon on your hair and it picks up charge: two different materials exchange electrons upon contact and separation, creating a potential difference. In triboelectric nanogenerators, micro-structured surfaces and pairing of materials enhance this effect to produce measurable currents.
In the RMIT implant, a flowing liquid—such as saline or blood—slides across the diamond–titanium surface, causing local charge interactions between the fluid and the hybrid material. Because diamond and titanium differ in electron affinity, charge transfer can occur under flow.
The Tribovoltaic Effect
The tribovoltaic effect occurs at semiconductor interfaces. Under mechanical friction, charge carriers are driven across the interface, generating a direct current. Because diamond (when doped) can act as a semiconductor, friction between the fluid and diamond–titanium interface can drive electron flow without requiring full contact-separation cycles.
This mechanism often yields lower impedance and higher current density compared to pure triboelectric designs. Combined, the two effects allow the implant to convert the motion of bodily fluids into continuous electrical output.

Why Diamond and Titanium Are the Perfect Pair
The choice of materials is central to the success of this design.
- Titanium is a well-established implant material: strong, lightweight, corrosion-resistant, and highly biocompatible. It acts as the structural backbone.
- Diamond is chemically inert and extremely durable. When doped (e.g. with nitrogen or boron), diamond can exhibit semiconductive properties.
By embedding diamond particles inside titanium, the composite becomes an active, multifunctional platform. Dr. Ahnood notes the diamonds “transform titanium from a passive structural material into one capable of scavenging energy, sensing flow, and receiving wireless power.”
The resulting hybrid remains durable and biocompatible, while improving cell adhesion and reducing bacterial adhesion. Surface analysis by the research team confirmed that the diamond particles remain stably embedded within the titanium matrix.
3D Printing with Laser Metal Deposition
To manufacture the implant, the researchers used a TRUMPF TruLaser Cell 7020 system via a process known as laser metal deposition.
A powder mixture of titanium and diamond (commonly a 30:70 diamond-to-titanium ratio) is fed into a laser focus. The laser melts the titanium, which encapsulates the diamond particles, building the structure layer by layer. After printing, microscopic analysis confirmed that the diamond particles remained intact and well distributed within the titanium matrix.
Professor Kate Fox of RMIT emphasized a key advantage: this method allows the fabrication of patient-specific implant geometries that combine mechanical support with sensing and energy functionality.
Wireless Energy Through Tissue
Harvesting energy from fluid flow is promising, but not always sufficient. To support greater energy demands, the implant also allows wireless power transfer via non-radiative capacitive coupling.
In this scheme, conductive plates on opposite sides of the tissue form a capacitor. Oscillating voltages applied externally drive displacement currents through the tissue, which generate current in the implant’s conductive elements.
The RMIT team demonstrated powering an LED through a 5 mm tissue-equivalent layer at 20 MHz. They also fabricated resonant coils (~825 MHz) and drove them at 1.14 GHz, producing a 3 °C temperature rise—a potential avenue for controlled heating in drug release or therapy.
This external wireless link can supplement harvested energy from fluid flow, enabling dual-mode power management.
Real-World Applications
There’s a wide range of potential applications for this innovation:
- Smart stents / vascular implants: monitor blood flow in real time and detect blockages autonomously.
- Neural interfaces / brain stimulators: provide electrical recording or stimulation using integrated conductive surfaces.
- Drug-release systems: use local heating or electrical triggering to release therapies precisely.
- Prosthetics / bone scaffolds: embed sensors or feedback within load-bearing implants.
- Industrial sensing in remote or harsh environments: where replacing batteries is impractical.
Although these applications remain largely speculative at present, they point to a future of self-powered, intelligent implants.
Challenges and the Road Ahead
While the results are promising, many hurdles remain:
- Power Output & Efficiency: Current electrical output is modest. The team must improve surface design, diamond doping, and geometry.
- Long-Term Biocompatibility & Stability: Implants must withstand biological conditions, mechanical stresses, and immune responses over time.
- Wireless Safety & Heating: Energy transfer must avoid harmful heating of tissue; regulatory constraints on electromagnetic exposure will apply.
- Integrating Electronics: Devices must include ultra-low-power electronics that can operate within the limited energy budget.
- Manufacturing & Scalability: Ensuring consistent material quality, defect reduction, and cost-effective production is crucial.
- Regulatory & Clinical Testing: Before human use, the technology must pass rigorous in vitro and in vivo testing, then safety and regulatory approval.
The RMIT team is actively seeking collaborations with medical device manufacturers and translational partners to move this technology from the lab toward real-world applications.
The Future of Battery-Free Implants
The RMIT diamond–titanium hybrid marks a shift: from passive structural materials to active, energy-managing biomaterials. By harvesting both bodily fluid motion and external wireless energy, implants might one day operate autonomously—without battery replacements, fewer surgeries, and greater longevity.
As Dr. Ahnood put it, the vision is for implants that last a lifetime. The human body itself becomes the power source.
References
- RMIT University News – “Diamond power could be a medical implant’s best friend”
- Advanced Functional Materials – “Additively Manufactured Diamond for Energy Scavenging and Wireless Power Transfer in Implantable Devices”
- 3D Printing Industry – “RMIT research points to diamonds as ‘medical implant’s best friend’”
- VoxelMatters – “RMIT researchers 3D print diamond–titanium implants”
- Electronics Online – “3D‑printed diamond device powers medical implants”